FGF23 & Hypophosphatemic Rickets/Osteomalacia
FGF23
FGF23 is the lastly identified FGF family member and one of the so-called enodocrine FGFs. FGF23 is produced in osteoblast/osteocyte and secreted into the blood to target the renal proximal tubular, where FGF23 suppress the reuptake of serum Phosphate (Pi) and concurrently inhibit the activation of 1alpha hydroxyvitamin D. Modulation of these two passages ultimately lead to down-regulation of the serum Pi level (Fig.1). Following researches, as a whole, demonstrated that FGF23 is the master regulator of the serum Pi level.
After the identification of FGF23 as the key Pi regulating molecule, part of the congenital and acquired hypophosphatemic diseases have been revealed to be occurred as the consequence of the inappropriately excess of serum FGF23 level (>>>FGF23 & Hypophosphatemic Rickets/Osteomalacia). In addition to that, it has been already clarified that FGF23 is subjected to the enzymatic cleavage as the part of the regulating mechanism of serum FGF23 level (Fig.2) and that the molecule Klotho which has been believed to be related to the senescence is essential for the signal transduction of FGF23 in the target cells (Fig.3).
Among the hypophosphatemic rickets/osteomalacia caused by the excess action of FGF23, it is supposed that a paraneoplastic syndrome; Tumor induced osteomalacia cases were often misdiagnosed because the identification of the responsible tumor is often times very hard because of the small tumor size or the diversity of the tumor origin in location.
In collaboration with a pharmaceutical company, we developed a ELISA for FGF23 to discriminate the hypophosphatemia due to FGF23 excess from the other causes. In our lab, FGF23 measurement is regularly performed to determine the pathogenesis of the cases or in the part of the basic researches. Please contact us in case there is a sample, FGF23 level in which is of interest (>>FGF23 Assay).
Also we are investigating the clinical usefulness of the systemic venous sampling of FGF23 in order to localize FGF23 producing tumor in Tumor induced osteomalacia patient. Please feel free to contact us about the referral of the patient (>>>Octreoscan and Venous FGF23 Sampling in TIO)。
In our lab, clinical investigations related to FGF23 mentioned above are underway. As well, we are engaging in the basic researches to clarify the precise regulating mechanism of serum FGF23 level and the sensing mechanism of serum Pi level on the basis of the data we obtained from these clinical researches.
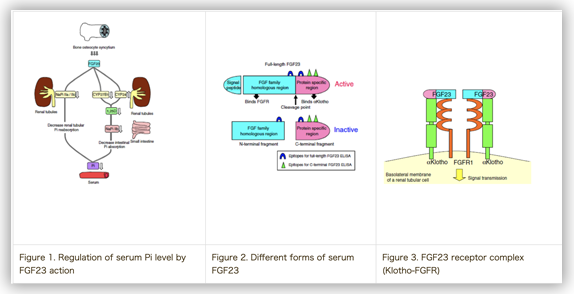
Fig.1-3 from Ref.6 (Ito N, et al. Clin Rev Bone Miner Metab 2014)
FGF23&Hypophosphatemic Rickets/Osteomalacia
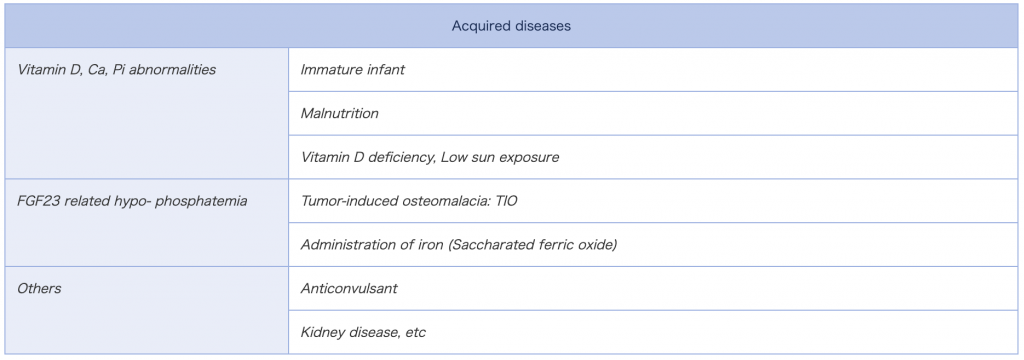
After identification of FGF23 as the master serum Pi regulator, part of the hypophosphatemic diseases have been revealed to be attributed to the excess of FGF23. In table.1 at the bottom, etiology of FGF23 related hypophosphatemic rickets/osteomalacia and the other hypophosphatemia are listed. In the FGF23 related hypophosphatemic rickets/osteomalacia, relatively high FGF23 level is observed. In our lab, FGF23 level is regularly assayed to investigate pathophysiology of the diseases with disturbed Pi levels. Please feel free to contact us if you have samples of interest (>>>FGF23 Assay).
Also we analyze the causative genes of some congenital hypophosphatemic diseases (ADHR, XLHR, ARHR1-2, HHRH). Please contact us about this gene analysis, as well.

FGF23 Assay
Diseases
Diseases in which FGF23 assay might be worthwhile are hypophosphatemic diseases. Please refer to Table.1 (>>>FGF23 & Hypophosphatemic Rickets/Osteomalacia). Also the other hypophosphatemia without any known cause is ideal to be subjected to FGF23 measurement,
Chronic kidney disease and hypophosphatemia after kidney transplantation should be excluded because serum FGF23 levels are consistently high in these diseases.
Request form
1 Please contact us by e-mail to request FGF23 assay and let us know the number of samples beforehand.
Yuka Kinoshita: yukarin0811@gmail.com
or
Nobuaki Ito: nobitotky@gmail.com
2 We will reply to the e-mails at the earliest stage possible. After the confirmation of our reply, please send the frozen serum or plasma samples of 1ml to the address below. Would you make sure to specify the date of delivery on weekday?
Please also send us an e-mail again with the request form attached.

Please fill the gray area in the request form. There is example on the second sheet.
Other important information might be written in the comment column.
All the measurements listed are not necessarily filled up.
FGF23 measurements will be informed usually within two months.
Please let us know in the case if the early measurement is urged.
The assay will be performed at our expense.
Mailing address
Yuka Kinoshita, Nobuaki Ito
Department of Endocrinology & Nephrology, University of Tokyo Hospital
7-3-1, Hongo, Bunkyo-ku, Tokyo 113-8655 JAPAN
Attention
Before you send us samples, please refer and conform to the ethical code of the facility. If needed, please gain an approval from the institutional ethical committee beforehand and obtain a written consent from the patient or the parent of the patient.
Personal data of the patient are not necessarily to be send to us, although age and gender column should be filled without any exception as reference range of the serum phosphate differ s according to the age and an X-linked recessive disorder is included in the differential diagnosis.
Octreoscan and venous FGF23 sampling in TIO
Hypophosphatemic tumor induced osteomalacia:TIO is one of the paraneoplastic syndromes, which is caused by the FGF23 producing tumor.
The prevalence of this disease has been assumed to be very rare (one in 100,000 to 1,000,000). However after the identification of FGF23 as the responsible humoral factor in TIO in 2001 and the development of the FGF23 assay, the number of the case report of TIO rapidly increased. Nowadays the actual prevalence of TIO is presumed to be much higher than that was expected before.
Clinical presentation of adult onset TIO includes bone pain, muscle weakness, trouble in walking, fracture and pseudofracture. As the measurement of serum phosphate is not routinely performed in outpatient booth, most of the patients who only present general bone pain, muscle weakness and trouble in walking are misdiagnosed as some neuromuscular diseases or fibromyalgia or other collagen diseases or psychogenic disorder in some cases.
To accurately diagnose TIO, first, serum Pi should be measured. Then serum FGF23 measurement ought to be performed. Finally exclusion diagnosis of hereditary FGF23 related hypophosphatemic rickets/osteomalacia is required to make a diagnosis of TIO.
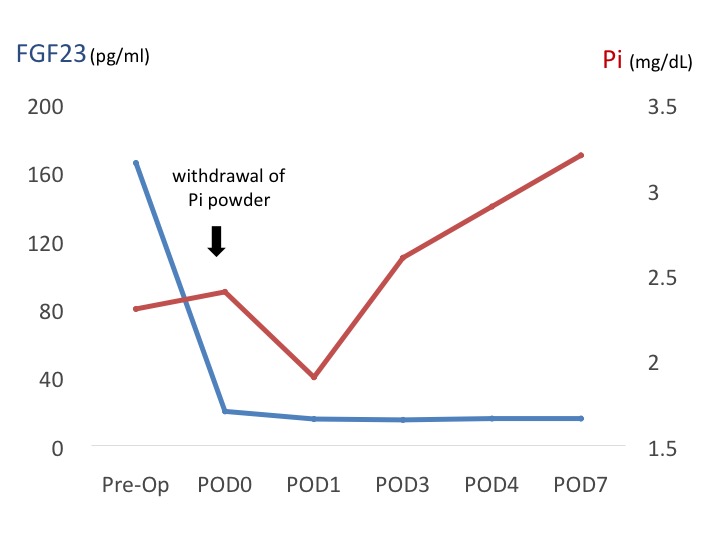
After the successful removal of the responsible tumor, serum FGF23 and Pi level return into the normal range, and the part of the symptoms and phenotypes of rickets/osteomalacia will be improved. However, the identification of the responsible tumor is often problematical, because the responsible tumor could arise anywhere in the body (bone: about 50%, soft tissue: about 50%). Also, size of the responsible tumor is frequently smaller than 10 mm. Furthermore, Identification of nodules by CT, MRI or PET in TIO patients don’t always suggest that the revealed nodules produce excess FGF23. Therefore, the other study is required to validate the overproduction of FGF23 by the detected nodule, as the next step.
Recently, the functional and localizing studies for the productivity of FGF23 contain Octreoscan (111In-pentetreotide) and 68Ga-peptides(DOTATOC, DOTATATE, DOTANOC),and systemic venous FGF23 sampling which our lab has developed. Every method mentioned above bedisides Octreoscan is off-label use in Japan.
Among the scintigram studies, 68Ga-DOTATOC is currently reported to be highly sensitive for the identification of FGF23 producing tumor. However, 68Ga-DOTATOC is still not the method which is capable to directly confirm the over production of FGF23. Also there are false-positive accumulation of isotope in fracture and pseudofracture lesion which is often occurred in TIO patients.
In contrast, by performing the systemic venous sampling, the excess production of FGF23 in the culprit tumor can be directly confirmed. In our hospital, systemic venous FGF23 sampling is operated with catheter inserted from the inguinal vein and the serum samples are taken from 20 to 30 different vein trunk and branches in the whole body. If there is an already known tumor which is revealed by CT, MRI or the above mentioned scintigram studies the step up of serum FGF23 level near the tumor could be a very potent supportive data for the FGF23 production of the tumor. In the case that there is no candidate tumor, the step up of FGF23 could be a strong clue to find the culprit tumor by consecutively operated detailed imaging studies. Patient have to be hospitalized for 3 days and experience the catheterization study for a few hours. Although the sampling might be a little more physically invasive than scintigram studies, specificity of the study is higher than that.
In 2016, as Octreoscan was approved to be a part of health insurance treatment, current strategy to identify the FGF23 secreting tumor is as follows.
① Tumor localization by Octreoscan, PET-CT, whole body CT, whole body MRI.
② Systemic venous FGF23 sampling from 20 points. If there are some localized tumor in some imaging test, set the additional sampling points around the possible culprits.
③ Identify the FGF23 secreting tumor when the result of FGF23 sampling is compatible with the Octreoscan finding. If there is no evident tumor revealed by the imaging methods, scrutinize the images mainly of Octreoscan around the FGF23 elevated points in FGF23 sampling.
In our facility, FGF23 secreting tumor identification steps as stated above is strongly recommended for the all patients supposed to be suffering from TIO. Systemic venous FGF23 sampling in addition to functional diagnostic scintigram should be performed especially in the case with the culprit tumors existing in the region where the resection of the tumor could cause considerable functional impairment or cosmetic disturbance (e.g. bone tumor included in a joint, soft tissue in the face) or the tumor with the expected high risk of the surgery (e.g. intracranial tumor).
Also the systemic sampling is comparatively recommended in the clearly acquired hypophosphatemic case without any tumor identified by scintigram.
In our hospital, systemic venous FGF23 sampling is conducted as the clinical study, which means the suspected TIO patient doesn’t have to incur cost for this test.
If there is suspected TIO case and the operation is considered as the treatment option, please feel free to contact us for consultation about the procedure of Octreoscan and systemic FGF23 venous sampling.
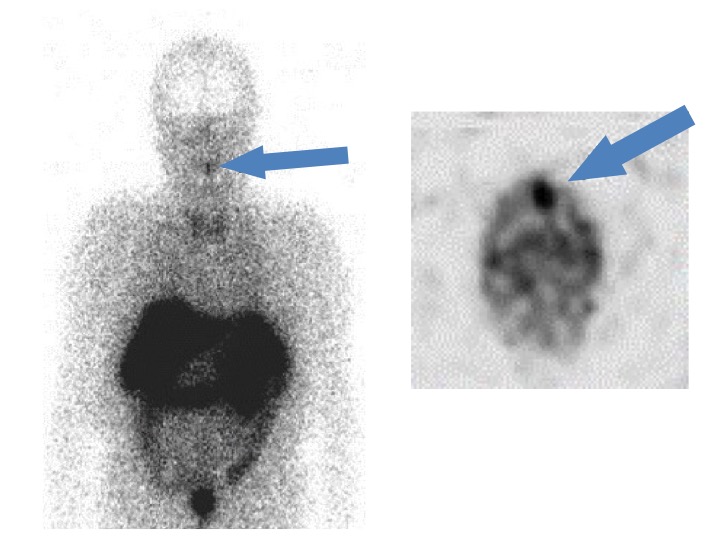
Brief presentation of the TIO case is shown below with the causative tumor in the alveolar bone which is not localized by the common imaging tests (CT, MRI), however the tumor was localized and functionally identified by the Octreoscan and systemic FGF23 venous sampling in our facility.
58 yo male
He was suffering from systemic bone pain and walking difficulty for 14 years and was misdiagnosed as ankylosing spondylitis. 9 years ago, his serum FGF23 level was revealed to be elevated (120 pg/ml, reference range 10~50 pg/ml), indicating the actual diagnosis of TIO, although there was no identified tumor by CT and MRI. The patient was treated by active vitamin D and inorganic phosphate powder, which enabled him to walk, however his activity was substantially restricted because of the bone pain and muscular weakness.